Silicates
 The first silicate products, such as a pottery, were obtained at the down of civilization. The age of the earliest fragments of man-made burnt clay, according to archeologists, is about 15 century BC, whereas the first industrial pottery has been produced in Egypt in 5000 BC. Since that time ceramics provides man with tools, durable containers and even a roof. Glassmaking came a little later, about a third century BC. Despite the significant age, silicate industry is successfully growing up till now. Since silicates are the most common raw material in nature (Earth's crust consists of 75 % aluminosilicates, and 12% silica which are represented by more than 500 types of minerals), it is not surprising that silicate products are inherently woven into human life. A large number of man-made silicates exist nowadays: Inorganic binders like cement and water glasses, catalysts made of synthetic zeolites, organosilicate compounds such as tetraethyl orthosilicate (TEOS) which is commonly used as a precursor in the sol-gel processing, etc.
The first silicate products, such as a pottery, were obtained at the down of civilization. The age of the earliest fragments of man-made burnt clay, according to archeologists, is about 15 century BC, whereas the first industrial pottery has been produced in Egypt in 5000 BC. Since that time ceramics provides man with tools, durable containers and even a roof. Glassmaking came a little later, about a third century BC. Despite the significant age, silicate industry is successfully growing up till now. Since silicates are the most common raw material in nature (Earth's crust consists of 75 % aluminosilicates, and 12% silica which are represented by more than 500 types of minerals), it is not surprising that silicate products are inherently woven into human life. A large number of man-made silicates exist nowadays: Inorganic binders like cement and water glasses, catalysts made of synthetic zeolites, organosilicate compounds such as tetraethyl orthosilicate (TEOS) which is commonly used as a precursor in the sol-gel processing, etc.
The term "Silicates" can be defined as compounds containing [SiO4]4- anions. However, the silicon atoms in silicates may exist with higher coordination numbers than four, for example six as in the case of Stishovite (high-pressure polymorph of SiO2). Additionally, oxygen atoms may be replaced by fluorine atoms, as in the case of hexafluorosilicates, salts of hexafluorosilicic acid (H2SiF6). There are numerous minerals containing different silicon-oxygen combinations that can be found in nature. Most of the natural silicates, such as micas, feldspar, Beryl, Wollastonite, etc. are formed by the solidification of magma (igneous origin). Some silicates are also formed in metamorphic rocks such as schists and gneisses. Additionally, clay minerals such as Kaolinite (Al2Si2O5(OH)4) or Montmorillonite ((Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2nH2O) are exogenous because they were formed by weathering of primary (endogenous) rocks. Natural silicates can exist in both crystalline and amorphous states. One of the typical examples of silicate minerals, containing amorphous silica, is precious opal which consists of silica particles and silicic acid xerogel as a binder.
The Main Structural Unit
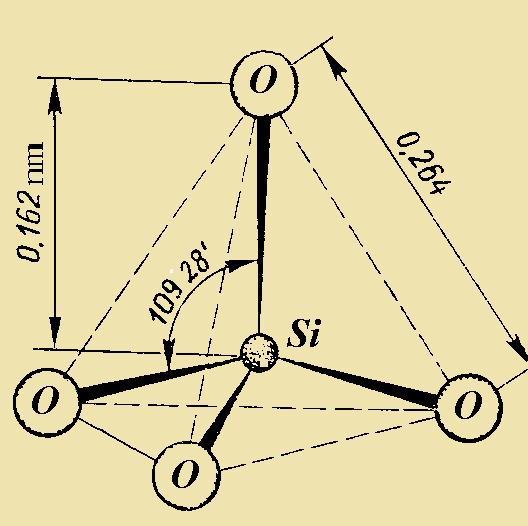 The main structural unit of silicates is a tetrahedral cluster containing one silicon atom and four oxygen atoms. The size of [SiO4]4- tetrahedral is relatively stable, with Si-O bond length varying from 0.161 to 0.164 nm at ambient conditions. The strength of S-O bonds is pretty high (the dissociation energy is ~ 452 KJ/mol) that provides the thermal stability and chemical resistance of the majority of silicate compounds. The tetrahedral clusters can be polymerized i.e., linked to each other through the bridging oxygen atoms. They are able to form polymers by means of linkage with one, two, three or four neighboring tetrahedra, forming siloxane Si-O-Si bonds. Other ions can be located in the silicate lattices such as to Lithium (Li+), Sodium (Na+), Potassium (K+), Beryllium (Be+), Magnesium (Mg2+), Calcium (Ca2+), Zinc (Zn2+), Boron (B3+), Aluminum (Al3+), Beryllium (Be2+), (F-) etc. as well as ions of Titanium, Manganese, and Iron in various oxidation states. Some cations such as Aluminum, Boron, Berryllium are able to isomorphically substitute silicon atoms in the silica-oxygen tetrahedra. However, most of them are located out of the anionic framework and play the role of "charge balanced cations". They are usually six-coordinate.
The main structural unit of silicates is a tetrahedral cluster containing one silicon atom and four oxygen atoms. The size of [SiO4]4- tetrahedral is relatively stable, with Si-O bond length varying from 0.161 to 0.164 nm at ambient conditions. The strength of S-O bonds is pretty high (the dissociation energy is ~ 452 KJ/mol) that provides the thermal stability and chemical resistance of the majority of silicate compounds. The tetrahedral clusters can be polymerized i.e., linked to each other through the bridging oxygen atoms. They are able to form polymers by means of linkage with one, two, three or four neighboring tetrahedra, forming siloxane Si-O-Si bonds. Other ions can be located in the silicate lattices such as to Lithium (Li+), Sodium (Na+), Potassium (K+), Beryllium (Be+), Magnesium (Mg2+), Calcium (Ca2+), Zinc (Zn2+), Boron (B3+), Aluminum (Al3+), Beryllium (Be2+), (F-) etc. as well as ions of Titanium, Manganese, and Iron in various oxidation states. Some cations such as Aluminum, Boron, Berryllium are able to isomorphically substitute silicon atoms in the silica-oxygen tetrahedra. However, most of them are located out of the anionic framework and play the role of "charge balanced cations". They are usually six-coordinate.
Crystalline Silicates
There are several classifications systems of crystalline silicates. The most comprehensive one was developed by Machatschki and Bragg in 1930s. However, the range of determined silicate structures has been expanded since that time and some minerals do not fall into Machatschki and Bragg classification. Now the most commonly used classification system takes into considerations the degree of polymerization of the [SiO4] -4 tetrahedral.
 Nesosilicates (orthosilacates) are silicates with isolated (not polymerized) [SiO4]4- clusters. The negative charge of the anions is neutralized by the cations which were listed above. The typical examples are the olivine's group minerals: Forsterite (Mg2SiO4) and Fayalite (Fe2SiO4), wherein Mg2+ and Fe2+ ions exist in 6-coordinated state and form [MeO6] groups. The structure of Monticellite (MgCaSiO4) is very similar to Forsterite, but the difference is that a half of the total amount of Mg2+ ions is substituted by Ca2+. Kyanite, Sillimanite and Andalusite which have the same composition (Al2SiO5) and different lattices, as well as Mullite (3Al2O32SiO)2 are representatives of ortho- aluminosilicates. They all contain [AlO6]3- groups which are linked in chains along the C direction. These chains are connected by the individual [SiO4]4- tetrahedral. Thus, there are Al-O-Al and Si-O-Al bonds, but there are no Si-O-Si bonds in this type of aluminosilicates.
Nesosilicates (orthosilacates) are silicates with isolated (not polymerized) [SiO4]4- clusters. The negative charge of the anions is neutralized by the cations which were listed above. The typical examples are the olivine's group minerals: Forsterite (Mg2SiO4) and Fayalite (Fe2SiO4), wherein Mg2+ and Fe2+ ions exist in 6-coordinated state and form [MeO6] groups. The structure of Monticellite (MgCaSiO4) is very similar to Forsterite, but the difference is that a half of the total amount of Mg2+ ions is substituted by Ca2+. Kyanite, Sillimanite and Andalusite which have the same composition (Al2SiO5) and different lattices, as well as Mullite (3Al2O32SiO)2 are representatives of ortho- aluminosilicates. They all contain [AlO6]3- groups which are linked in chains along the C direction. These chains are connected by the individual [SiO4]4- tetrahedral. Thus, there are Al-O-Al and Si-O-Al bonds, but there are no Si-O-Si bonds in this type of aluminosilicates.
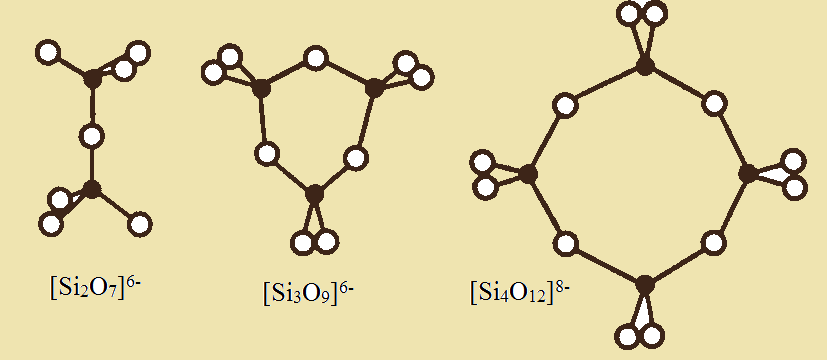
 Sorosilicates are silicates containing [Si2O7]6-clusters. The sharing of one oxygen atom between two neighboring tetrahedra results in formation of a dimer. This type of structure is realized, for example, in Akermanite (Ca2Mg[Si2O7]), Rankinite (Ca3[Si2O7]) and in Thortveitite ((Sc,Y)2Si2O7).
Sorosilicates are silicates containing [Si2O7]6-clusters. The sharing of one oxygen atom between two neighboring tetrahedra results in formation of a dimer. This type of structure is realized, for example, in Akermanite (Ca2Mg[Si2O7]), Rankinite (Ca3[Si2O7]) and in Thortveitite ((Sc,Y)2Si2O7).
Cyclosilicates are silicates with cyclic clusters of three [Si3O7]6-, four [Si4O12]8-, or six [Si6O18]12- tetrahedra. The typical representative of cyclosilicates are Benitoite (BaTi[Si3O9], Beryl (Be3Al2[Si6O18]) and Cordierite (Mg2Al3[AlSi5O18]) containing six-membered ring clusters [AlSi5O18] in which one Si atom is substituted to Al.
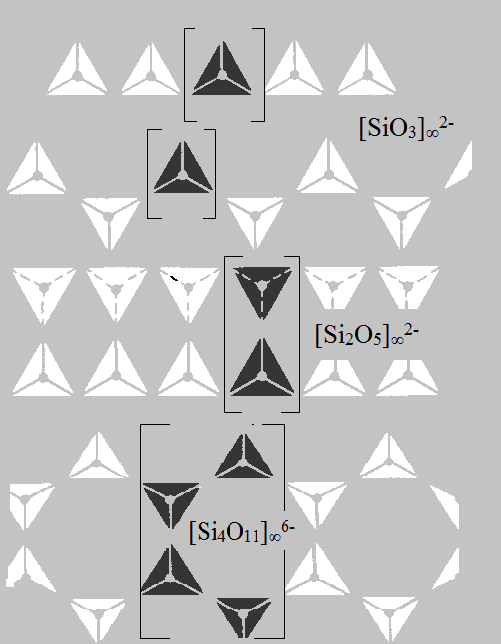
Inosilicates are silicates which consist of one-dimensional [SiO3]∞2-, [Si2O5]∞2- and [Si4O11]∞6- chains or ribbons and charge balanced ions. Enstatite (Mg2[Si2O5]2) and Diopside (CaMg[SiO3]2) are examples of chain silicates In inosilicates all tetrahedra have two common oxygen atoms and two excess negative charges for cations which connect these chains to a framework. The length of these chains is defined by size of the crystal.
Phyllosilicates (layered silicates) are silicates with two-dimensional layers of [SiO4]4- tetrahedral sharing three oxygen atoms between each other. Such structures provide a good cleavage, i.e. the ability to split along definite smooth planar surfaces. For example, micas, Talc (Mg3[Si2O5]2OH2), Kaolinite (Al2[Si2O5](OH)4) have a perfect cleavage along the crystal plain in the [001] direction. A substitution of silicon atoms for aluminum atoms occurs very often in anionic frameworks of layered silicates.
Tectosilicates (framework silicates) have a three dimensional framework where all four oxygen atoms of each tetrahedral are shared by the adjacent tetrahedra. If there is no Si atom substitution occurs, there will not be an excess negative charge in the silica-oxygen framework. That is why lattices of such tecstosilicates as silica (SiO2) and all its polymorphs (quartz, tridymite, cristobalite, coesite, stishovite) do not contain any cations. There are other types of tectocilicates as well, for example, in Phenakite's (Be2SiO4) structure [SiO4] tetrahedra are linked with [BeO4] tetrahedra and they form a three-dimensional framework together. In the case of feldspars SI4+ cation is replaced by Al3+ in tetrahedra providing negative charge of this anionic framework. This negative charge is balanced by cations e.g. Na+ in Albite (NaAlSi3O8), K+ in Microcline (KAlSi3O8) and Ca2+ in Anorthite (CaAl2Si2O8). Zeolites can also be attributed to tectosilicates [1].
Non-crystalline natural silicates
 Natural amorphous silica such as Diatomite and precious opal (natural hydrated amorphous silica) are examples of this type of non-crystalline silicates. They are amorphous in the sense that they does not give a sharp X-ray diffraction pattern but in some cases they have been shown to contain submicroscopic crystalline areas. Amorphous silica can be formed by condensation from the vapor phase ejected in volcanic eruptions or by deposition from saturated solutions. Silica is often contained in primitive organisms, plants, or diatoms (diatomite) and remains in the Earth's crust in an amorphous state after their death. Opals have a structure that has been formed from uniform particles of colloidal silica 100-500 nm in diameter. The surface of these particles is highly hydrated, i.e. covered by OH groups. Opals may contain from 6 to 24 % water. The formation process of precious opal consists of three stages: 1) formation of uniform particles, 2) their regular deposition, 3) gluing them together. In nature, the process of making an opal takes a very long time [2].
Natural amorphous silica such as Diatomite and precious opal (natural hydrated amorphous silica) are examples of this type of non-crystalline silicates. They are amorphous in the sense that they does not give a sharp X-ray diffraction pattern but in some cases they have been shown to contain submicroscopic crystalline areas. Amorphous silica can be formed by condensation from the vapor phase ejected in volcanic eruptions or by deposition from saturated solutions. Silica is often contained in primitive organisms, plants, or diatoms (diatomite) and remains in the Earth's crust in an amorphous state after their death. Opals have a structure that has been formed from uniform particles of colloidal silica 100-500 nm in diameter. The surface of these particles is highly hydrated, i.e. covered by OH groups. Opals may contain from 6 to 24 % water. The formation process of precious opal consists of three stages: 1) formation of uniform particles, 2) their regular deposition, 3) gluing them together. In nature, the process of making an opal takes a very long time [2].
Aqueous Silicates
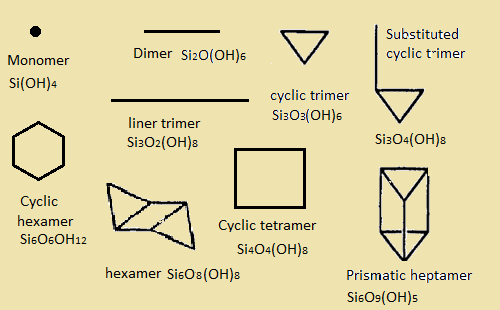 The dissolution of silica involves a chemical reaction of hydrolysis: SiO2 + H2O → Si(OH)4. It is well known that, the solubility of silica in water under normal conditions is very low: The equilibrium concentration of amorphous SiO2 in water at 25℃ is 70 ppm, whereas for crystalline silica, such as quartz, this value is no more than 6 ppm. In order to obtain a supersaturated solution of silica, an elevated temperatures, pressures and a high pH (around 12), should be used [2] The supersaturated solutions are unstable and susceptible to condensation under ambient conditions. Even above pH 7 silicic acid is deprotonated to a high extent and the anion species have a high (negative) surface charge. In the presence of alkaline metals such as Sodium (Na) or Potassium (K) these systems consist of hydrated cations and polysilicic anions (products of polycondensation process) (see eq):
The dissolution of silica involves a chemical reaction of hydrolysis: SiO2 + H2O → Si(OH)4. It is well known that, the solubility of silica in water under normal conditions is very low: The equilibrium concentration of amorphous SiO2 in water at 25℃ is 70 ppm, whereas for crystalline silica, such as quartz, this value is no more than 6 ppm. In order to obtain a supersaturated solution of silica, an elevated temperatures, pressures and a high pH (around 12), should be used [2] The supersaturated solutions are unstable and susceptible to condensation under ambient conditions. Even above pH 7 silicic acid is deprotonated to a high extent and the anion species have a high (negative) surface charge. In the presence of alkaline metals such as Sodium (Na) or Potassium (K) these systems consist of hydrated cations and polysilicic anions (products of polycondensation process) (see eq):
≡Si - OH + OH- → ≡ SiO- + H2O
≡SiO- + HO-Si → ≡Si - O - Si≡ + OH-
The anion species can be represented by monomers, oligomers, and polymeric three dimensional structures. The hydrated cations are involved in the formation of an electric double layer. The electric double layer and steric factor (the decreasing of monomers attachment probability to branched particles) are the causes of the aggregative stability of such systems [3]. Supersaturated solutions of silicic acid can be used in sol-gel processing as precursors along with the more widely used system: TEOS (TMOS)-H2O-ROH, where ROH is an alcohol. Aqueous silicate solutions can provide aerogels and xerogels or dense ceramics after solvent extraction, evaporation of solvent or heating respectively. One of the most widely used examples of alkaline silicates solutions is water glass. Commercial water glasses are concentrated (around 40 wt. % of silicate). Their composition can be expressed as: R2O●mSiO2●xH2O, where, R2O is sodium oxide or potassium oxide and m is the mole ratio silicon oxide to the alkaline metal oxide. Water glasses are applied in technology of acid-resisting concrete and other construction materials.
Crystalline, Semi-crystalline and Amorphous Synthetic Silicates
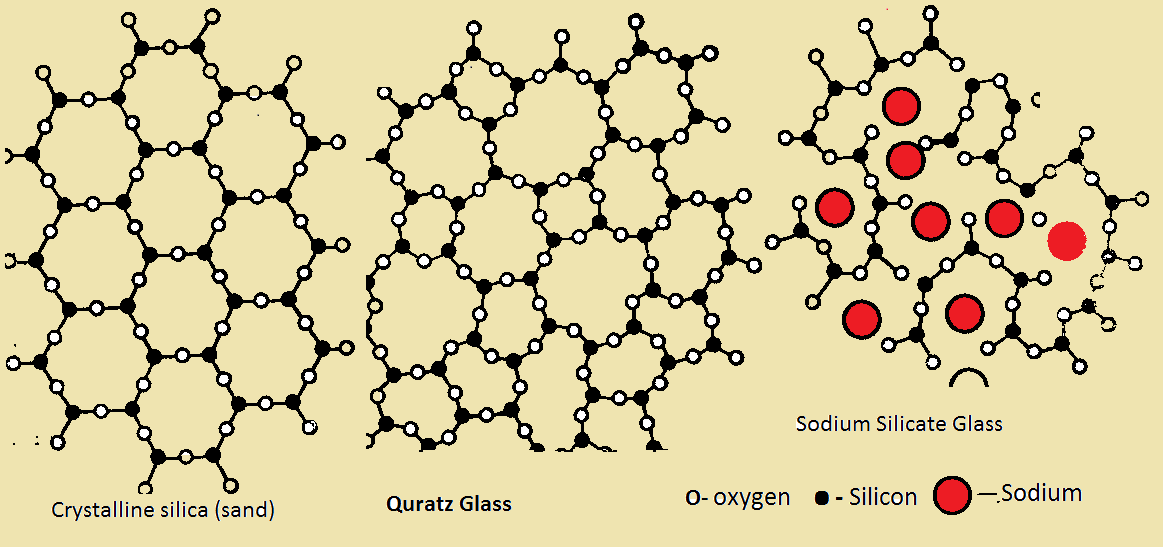 Cement and Ceramics: Ceramics and hydraulic cements are the major classes of crystalline synthetic silicates having a significant industrial importance. Both ceramics and cement are formed by fusion at high temperatures (1000-1600 C). Ceramics, in general, is highly crystalline substance, containing some "glassy phase". If the glassy phase is contained in amounts providing the crystallinity in the range from 30% up to 90%, then such composites are usually called Glass Ceramics. Portland cement is the most common cement which is widely used in constructing. It is formed by sintering of a mixture of natural clay, limestone (CaCO3), and sand (SiO2) as well as some additives, like iron oxide (Fe2O3). The sintering is carried out at temperatures ranging from 1400 to 16000 C. The product of this synthesis is a clinker (grains 3-25 mm in diameter) which consists of the four type of silicates, namely, Tricalcium Silicate (3CaO●SiO2), R-Dicalcium Silicate (R-2CaO●SiO2), Tricalcium Aluminate (3CaO●Al2O3) and Calcium Aluminoferrite (4CaO●Al2O3●Fe2O3), as well as free lime (CaO and Ca(OH)2) and free MgO. After subsequent cooling, these grains are mixed with gypsum and grinded together to obtain a powder which we usually call "cement". If we add water to the powder, the calcium silicate hydrates will be formed and the hardening process will occur [3].
Cement and Ceramics: Ceramics and hydraulic cements are the major classes of crystalline synthetic silicates having a significant industrial importance. Both ceramics and cement are formed by fusion at high temperatures (1000-1600 C). Ceramics, in general, is highly crystalline substance, containing some "glassy phase". If the glassy phase is contained in amounts providing the crystallinity in the range from 30% up to 90%, then such composites are usually called Glass Ceramics. Portland cement is the most common cement which is widely used in constructing. It is formed by sintering of a mixture of natural clay, limestone (CaCO3), and sand (SiO2) as well as some additives, like iron oxide (Fe2O3). The sintering is carried out at temperatures ranging from 1400 to 16000 C. The product of this synthesis is a clinker (grains 3-25 mm in diameter) which consists of the four type of silicates, namely, Tricalcium Silicate (3CaO●SiO2), R-Dicalcium Silicate (R-2CaO●SiO2), Tricalcium Aluminate (3CaO●Al2O3) and Calcium Aluminoferrite (4CaO●Al2O3●Fe2O3), as well as free lime (CaO and Ca(OH)2) and free MgO. After subsequent cooling, these grains are mixed with gypsum and grinded together to obtain a powder which we usually call "cement". If we add water to the powder, the calcium silicate hydrates will be formed and the hardening process will occur [3].
Glass: The modern definition of glass is an "amorphous solid completely lacking in long range, periodic atomic structure, and exhibit a region of glass transformation behavior", so any material formed by any technique (vapor deposition, sol-gel processing, or the melt-quenching technique), which exhibits glass transformation behavior is a glass and silica is not a required component of them. However, that glass we are used to have in our everyday life (windows, beer bottles, glasses, optical fibers in our telecommunication system, etc.) is silicate glass formed by cooling of a melt. This technology commonly uses quartz sand as a main source of SiO2 and alkaline- and alkaline earth- containing components. Homogeneous silicate melts of such composition can be obtained at temperatures around 1200 0C or more. The cooling process should be fast enough to avoid crystallization and to form transparent glass of a good quality. If the melt has a proper temperature-dependence of viscosity, the structure of the cooled glass will be similar to frozen liquid which characterized by the short range order [4].
Geopolymers is another example of artificial semi-crystalline materials. The name "Geopolymers" has been introduced by J. Davidovich and is applied to a wide range of alkaline - or alkali-silicate-activated aluminosilicate compounds. They can be obtained from amorphous clays and alkali-silicate solutions without any melting under high temperatures and avoiding significant CO2 emission. The hardening process of the alkali activated aluminosilicates is based on formation Si-O-Al and Si-O-Si bonds under ambient conditions. It is likely that these products have a highly significant commercial potential, but this technology is just being developed today [5].
Acknowledgement

Content of the web page has been developed by Taisiya Skorina, Sr. Materials Chemist at 3M Corporation. Read about her project at MIT.
References
[1] The Physical Chemistry of the Silicates by Wilhelm Eytel (University of Chicago press, 1954)
[2] The Chemistry of silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry by Ralph K. Iller (A Willey Interscience Publication, 1979)
[3] Introduction to Glass Science and Technology 2nd Edition by J.E. Shelby (The royal Society of Chemistry, 2005)
[4] Advanced in cement technology: Chemistry, Manufacture and Testing 2nd Edition by S.N. Ghosh (Tech Books International, 2002)
[5] John L. Provis, Grant C. Lukey, and Jannie S. J. van Deventer "Do Geopolymers Actually Contain Nanocrystalline Zeolites? A Reexamination of Existing Results" Chem. Mater. 2005, 17, 3075-3085
Publications of the page author
Aqueous alteration of potassium-bearing aluminosilicate minerals: from mechanism to processing
Alkali metal ion source with moderate rate of ion release and methods of forming
Ion exchange in amorphous alkali-activated aluminosilicates: Potassium based geopolymers
Alkali silicate binders: effect of SiO2/Na2O ratio and alkali metal ion type on the structure and mechanical properties
Functional materials from local and earth-abundant precursors: Scalable and cost- efficient synthetic approach
More about silicates
- Silicates at Wikipedia
- The Building Blocks of Inorganic Compounds
- Mineral Tutorial
- Mineral Gallery - The Silicate Class
- Silicate Structures
- Introduction to Lithophile Minerals for Planetary Scientists
- Silicate Minerals
- Structural classification of natural non-crystalline silicates
- Basic Clays and Clay Bodies
- Manufacturing Artificial Stones
- Basic Concrete and Cement Principles and Terminology Used in the Industry
- Moxie International's Tutorial on Concrete
- Geopolymer Institute
- Alkali silicate binders: effect of SiO2/Na2O ratio and alkali metal ion type on the structure and mechanical properties
- Ion exchange in amorphous alkali-activated aluminosilicates: Potassium based geopolymers
- Cement and art
