Carbon: Bridging Inorganic & Organic Worlds:
The stabilized diamond-like — graphite-like (graphine) — polymer-like hybrid carbon phases as a forerunner of new materials
Over the preceding half a century, the technology was extremely successful in reaching or even exceeding the individual records of natural organic materials, including synthesizing the most flexible and self-lubricant plastics, possessing the highest specific strength graphite fibers and carbon-carbon composites, as well as the hardest crystals of diamond.
However, the natural potential of carbon materials is far from exhaustion. More than in any other domain of technology, the researches in this area are inspired by Nature. The animate and inorganic worlds demonstrate the entire specter of records in both structural and surface-forming materials: from powerful internal skeleton of shark combining seemingly incompatible flexibility and rigidity, to almost weightless exoskeleton of insects fighting the aerodynamic laws; from super-stiff diamond to super-sensitive and flexible skin of woman’s face; from soft graphite used for writing to the brain software which knows what to write.
The combination of the superior opposite features being observable in the animate nature, remains an eluding goal. Perhaps, this is exactly that area, where the surface science and engineering may open a window into a new space of materials, initially the surface-modifying coatings, but potentially the broad scope of structural materials as well.
The "diamond-like" carbon (DLC), which could already celebrate its 50' jubilee, represents actually a hybrid form combining all three major chemical carbon-carbon bonds – "polymer-like" sp chains, "graphite-like" sp2 planes, and "diamond-like" sp3 tetrahedrons. It is difficult to define an absolute priority in DLC synthesis. Probably, many researchers in different countries started more or less successful initial experiments in the very beginning of 1960s (the author of this post – in 1962).
However, the results of those early works weren't published, partly because the first inhomogeneous unstable films were obviously unfeasible for any practical applications, partly, due to super-secret circumstances in the former USSR where publication of any seemingly "strategic" research results were strictly forbidden.
The first publication belongs to S. Aisenberg and R. Chabot (J. Appl. Phys., 42, 2953,1971). Over the following decades, DLC films have been deposited by a variety of methods from high-energy ion-molecular fluxes (typically, in the range of 102-104eV) in the forms of hydrogenated and hydrogen-free films. The hydrogenated DLC is a pore-free material possessing the specific gravity about ~2.0 g.cm-3, e.g. about ~0.57 with respect to the dense hard carbon phase (crystalline diamond). The metals may be introduced in DLC up to relatively high concentration, and the metal-containing conventional DLC films (Me-DLC) have been synthesized particularly by C. Weismantel and his school in Germany (Thin Sold Films, 96, 31, 1982). However the "diamond-like" carbon is a stress-stabilized material. Usually the comprehensive stress in hydrogenated DLC (a-C: H) films is in '10+' GPa range, and the stress increases in proportion to hardness. The hydrogen-free DLC (i-C) with density up to 3.1-3.2 g/cm3 is particularly hard representative of diamond-like family, but its stress is also particularly high. Although the lower stress may be achievable in principle, the energetically favored process of the bonds reconfiguration will generate the post-growth compressive stress. On one hand, this comprehensive stress limits the film thickness and may cause the lose of adhesion, on the other hand, DLC is thermally an unstable material which starts to convert into graphite and lose its desirable properties with prolonged exposure to temperatures roughly about 250oC. It is particularly important that the electrical conductivity of the pure DLC films dramatically increases from the very beginning of its graphitization, while Me-DLC loses its homogeneity due to metallic micro-crystals formation even at relatively low metal content. In spite of these problems and limitations, the thin and especially ultra-thin DLC and Me-DLC are promising for many areas of important applications. These areas include both protective coatings (corrosion-resistant coatings, coatings for engineering tribological applications, even protective coatings for turbine engine, bio-compatible coatings in medical implants and tool), and functional films in electronics, acoustic, high-energy physics devices, flat panel displays. Indeed, the number of scientific publications devoted to fundamental research and commercial applications of DLC during a relatively short period counts a few thousands. The significance of this tremendous work far exceeds specific applications of DLC films, and this brief scattering sketch just marks a few points of DLC development.
The current post is devoted to more complex stabilized carbon-based phases. The search for an effective approach to stabilization took 16 years from the above mentioned start in 1962: the first ultra-thin stabilized diamond-like films had been deposited in 1978. It took two more years to develop functional technology and the first applications in satellite electronics (1980) and three more years to reach the industrial level of technology and start broad applications from computer hard disks to medical implants, from the ocean depth to the open space.
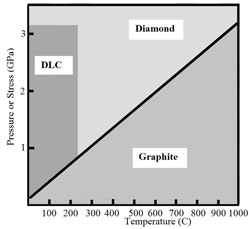
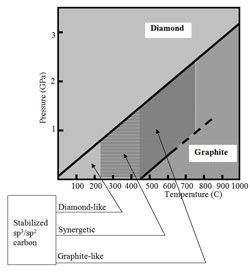
One may consider conventional DLC as the sp3: sp2 carbon stabilized by internal stress instead of external pressure, as it is shown in Fig. 1, a. Alternatively, the fine chemical stabilization shifts the carbon-diamond equilibrium, as it is shown in Fig. 1, b.
Fig. 1 Two major approaches to shift of the graphitediamond equilibrium in amorphous carbon: Diagram below (1, a) - high built-up intrinsic stress (DLC):
Next diagram (1, b) - chemical stabilization (diamond-like DLN, synergistic QUASAM and graphite-like phases):
Consequently, silicon-stabilized and silica-stabilized sp3: sp2 carbon are virtually stress-independent carbon phases. Typically, they possess stress < 0.1 GPa ( < 0.02 GPa, i.e. within the limits of characterization errors in many samples), long-term thermal stability 400-550oC, short-term thermal stability 550-850oC, hardness 15-30 GPa (56-57 GPa is maximum for micro-hardness, although in 'nano' scale the values not inferior to the crystalline diamond may be observed in some forms), fracture toughness 5-10 MPa√m (some samples >20 MPa√m). By varying the composition of the initial flux, one can achieve in the silicon-carbon, silica-carbon and metal-silica-carbon systems essentially the entire spectrum of relatively stable amorphous materials except of low silicon concentration in the vicinity of pure DLC.
As it is schematically shown in Fig. 2, amorphous phases in the C-Si-SiOx system may be classified into diamond-like carbon, stabilized by silica or silicon (1), silicon carbide and oxy-carbides (2), and amorphous silica and silicon, stabilized and reinforced by carbon (3). It is also shown an optimum chemical composition of silica-stabilized amorphous (DLN) and quasi-amorphous graphine-diamondlike (QUASAM) hard carbon phases (4). Except certain specific cases, the silica-stabilized carbon phases of this optimum composition are preferable.
Fig. 3 shows the stabilized amorphous quasi-phases in C-Si-SiO-Me system. The line corresponds to optimum composition of silica stabilized Me-C diamond-like composites of atomic scale.
Fig. 2 Stabilized C-Si-SiO quasi-phases:
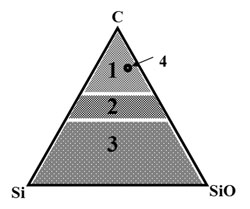
Fig. 3 Stabilized C-Si-SiO-Me quasi-phases:
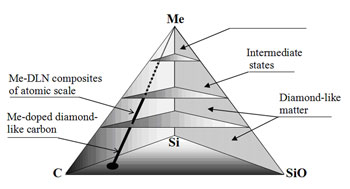
Fig. 4 Virtual continuum of quasi-phases in stabilized hybrid carbon:
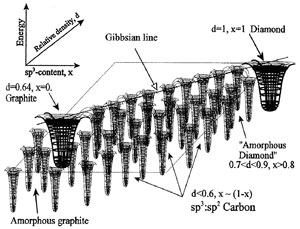
Some researchers suggest a general consideration of diamond-like nano-composites (DLN), conventional diamond-like carbon (DLC) and nano-crystalline diamond. Although such an approach is well justified, there is a principal difference between these three carbon matters. While the nano-crystalline diamond - a remarkable achievement in development of classical materials, DLC is a new carbon form combining some diamond and graphite features in one continuous solid matter. However, due to very basic nature of DLC, it may exist as the thin films only. Alternatively, a fine chemical stabilization of the hybrid sp3:sp2 carbon shifts the thermodynamic equilibrium and reveals a continuum of 'relatively-stable' carbon phases.
On one hand, while properly selecting the films growth conditions, most importantly – the incident beam energy, one may deposit a stabilized hybrid carbon matter with different proportion of sp, sp2 and sp3 bonds and correspondingly, with different properties, including the functionally gradient ones. On the other hand, the basic atomic arrangement of amorphous carbon phases depends mostly on substrate temperature. One of the unique peculiarities of these phases is an essential difference in their densities - from ~2.5 g/cm3 to nearly water density of relatively hard solid matters, from isotropic diamond-like DLN to synergetic slightly anisotropic quasi-amorphous graphene-diamond structure QUASAM to strongly anisotropic graphite-like carbon. The third carbon phase possesses strong cleavage and may not withstand even a moderate stress, although it may have important potential applications particularly due to its high thermal stability.
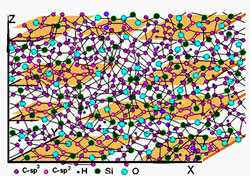
The higher substrate temperature during deposition, the higher is thermal stability of deposited structure. Furthermore, having been grown at certain temperature TG, the film may withstand a relatively long exposure up to temperature ~(TG+400). DLN is the subject of intensive researches and effective practical application over 35 years. The QUASAM technology is relatively young, the first material was produced in 1994-1995. But the superior mechanical and thermal properties of QUASAM combined with its lowest density, as well as high deposition rate and the energy saving technology proves its broad potential uses. Fig. 5 shows a model representation of silica-stabilized diamond-like carbon as an atomic-scale composite structure consisting of interpenetrating networks of atomic-scale: diamondlike carbon network and quartz-like silica network. Thus, this carbon phase should be considered as a composite of atomic scale. (The original term "Diamondlike Nanocomposites, or DLN" does not strictly correspond to these atomic-scale composite structures; however, it was used in various publications over quarter of century and should be preserved). Such a simplified model representation well describes t he DLN structures having been grown at low substrate temperature (~ 300, K) and a moderate beam energy (~1000 eV). The higher substrate temperature and incident beam energy during deposition, the higher density of inter-network bonds in the grown film. However, the C-C, Si-Si and Si-O bonds are predominant in DLN films having been grown in the entire range of growth conditions. Figure 6 shows a model representation of silica-stabilized quasi-amorphous carbon QUASAM. Oppositely to DLN, this is not a composite but strongly bonded structure. The major peculiarity of QUASAM structure represents its carbon atomic arrangement. The planes are shown for illustration purpose to indicate a predominant arrangement of sp2 bonds being mostly parallel to the substrate (the growth front). Although QUASAM does not reveal any azimuth orientation, it possesses one-axis anisotropy. This carbon phase consists of predominantly sp2 bonded graphite-like layers and predominantly sp3 bonded three-dimensional diamondlike framework. However, graphite-like layers in QUASAM structure are bonded together by the diamondlike framework. As the results, QUASAM combines high values of fracture toughness, hardness, as well as elastic modulus, its long-term temperature stability exceeds 500oC, while its density is in the range of 1.3 – 1.65 g/cm3.
Fig. 5 Model representation of DLN:
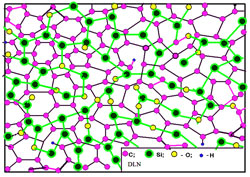
Fig. 6 Model representation of QUASAM:
Metals, in particularly transition metals, may be introduced in the silica-stabilized diamond-like matrix during its growth. Such co-deposition results with formation metaldielectric composites of atomic scale. This new kind of solid matter combines certain electronic features of both, metals and dielectrics over 18 orders of electrical conductivity at 300K and the most stable amorphous superconductors {W|C}, {Mo|C}, {Nb|C} at low temperature (Fig. 7).
Fig. 7 "Room temperature" conductivity(s300, S/m) with percolation transition and transition point TC to lowtemperature superconductivity vs. metal concentration X. Note two kinds of dependencies TC(X): monotonous - {Nb|C} and with maximum - {W|C} and {Mo|C}. A schematic diagram:
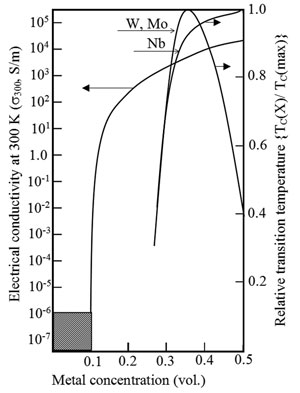
Fig. 8 Model representation of Metal-dielectric diamond-like composites of atomic scale:
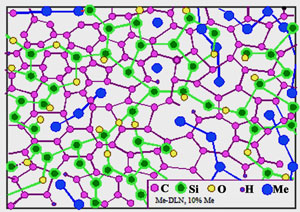
a
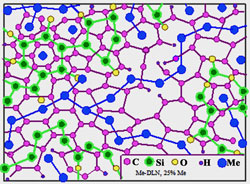
b
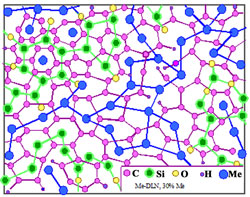
c
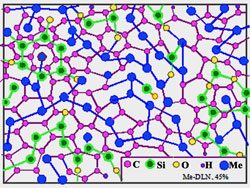
d
Fig. 9 Relative stability of Me-Carbon diamond-like composites of atomic scale:
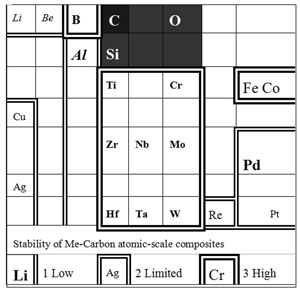
The maximum metal concentration Xmax (at.%) and temperature Tmax limiting such atomic-scale composite stability depends on atom radius and metal-matrix interaction. At present, evaluated ranges of stability are: 1.Low stability due to strong interaction: Li: T max < 300 K, Xmax ~25 at. %; Be, Al: Tmax ~300-400 K, Xmax ~30 at. %. Characteristic time of metal-dielectric composite degradation is about 10 h in the case of Li (by XPS) and ~103 h in the case of Al. In the last case the degradation is not so deep, but at X→30 at.% the Al-carbon composites are eventually losing adhesion and stripping. 2. A limited stability due to weak interaction: Cu, Ag, Au, Pt, Pd: Tmax ~700K, Xmax ~35-40 at. %. At room temperature the composites may be preserved during an unlimited time. However the "nanophases" of graphite and metals are forming at the annealing at T>Tmax . 3.The highest stability: Ti, Zr: Tmax ~ 700 K, Xmax ~40 at. %; Hf: Tmax ~ 800 K, Xmax ~35 at. %; Nb, Ta, Mo, W, Re: Tmax ~ 1000 K, Xmax ~40-45 at. %; Cr, Cr- Fe- Co- Ni alloys: Tmax >1000 K, transition to amorphous metals at X >50 at. %. In the case of hard-melting transition metals Fe, Ni, Cr, Mo, W, Hf, Nb, Ta thermal stability of the composite structure even exceeds stability of the diamond-like matrix.
The principle possibility to synthesize a diamond-like matter far apart from its thermodynamic stability is due to certain peculiarities of carbon nucleation. The variety and properties of carbon allotropic forms in small atomic aggregates are different from macroscopic ones. Consequently, macroscopic thermodynamics does not define the early stages of carbon nucleation unless a previously formed graphite substrate is introduced or graphite nuclei are formed. Due to its basic structure (cooperative interaction between the planes), graphite may exist in relatively large atomic aggregates only. For instance, a minimum graphite cluster holding at least one ring surrounded with the nearest completed coordination sphere should contain three layers of seven rings each, i.e. 72 atoms. A similar consideration in the case of sp3-tetrahedrons results with estimated minimum diamond clusters of 36 atoms.
Fig. 10 is a diagram 'Gibbsian energy vs. nuclei radius' plotted for carbon nucleation. The nucleus of new phase has to form the inter-phase boundary, and this positive contribution into Gibbs function creates a barrier for nucleation. When the supercooling (Δμ) exceeds some critical level, the barrier vanishes, i.e. even the smallest atomic aggregates become stable. Although real dependencies ΔG(R) in the range of small atomic groups aren’t smooth, for most elements they preserve this general character. However, while the stable carbon phase graphite exists in relatively large clusters only, various meso-molecular forms "fullerenes" may be formed as the result of three-dimensional nucleation. The minimum size of diamond and graphite as well as R-ranges of fullerenes are shown schematically. In the conditions of low or moderate supercooling, such as Δμ1, Δμ2 in Fig. 10, graphite is the only carbon phase that may be formed through homogenous mechanism of nucleation.
Fig. 10 Thermodynamic potential of the atomic groups vs. their radius for major carbon forms under different super-cooling conditio:
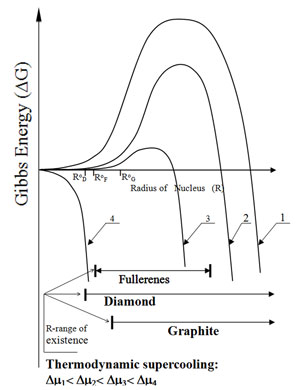
In a higher supercooling range around Δμ3 fullerenes become predominant allotropic forms. In a narrow supercooling range in the vicinity of Δμ4 diamond nuclei may be formed prior to both graphite and fullerenes. Depending on the energizing technique and substrate temperature, such homogeneous nucleation may result with crystalline diamond deposition or with deposition of a mixed solid matter, combining nanocrystalline diamond and amorphous "diamondlike" matter. Although such nano-composites possess certain useful properties for many practical applications, an interface material must be homogeneous near atomic-scale. Hence, an appropriate deposition technique for this purpose should minimize or exclude any homogeneous nucleation. During crystalline diamond deposition by CVD such nucleation may be prevented even at normal pressure due to chemical composition of gas phase. During deposition of the diamondlike matter, the homogeneous nucleation may be prevented using a collision-free flow of incident particles, i.e. pressure ≤10-2, Pa. Fig. 11 shows example of such equipment.
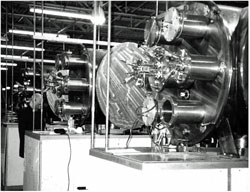
Fig. 11 Three identical deposition systems, each possessing 7 plasmatrons: six with internal diameter 160 mm and one with internal diameter 250 mm. The internal diameter of each chamber 1000mm. General design by the author of this post (Atomic-Scale Design, Inc., then New York); final design and manufacture by Energovac (Petersburg, Russia). This photograph made in 1996 in Long Island, NY. It was a unique and short period of close high-tech cooperation between the USA and Russia; as an active professional participant, I witnessed the mutually sincere readiness and openness of researchers, engineers and the scientific and industrial leaders from both sides.
The pure silica-stabilized diamond-like and synergistic matters, DLN & QUASAM, as well as Me-DLN diamond-like composites of atomic scale possess a number of the record-breaking properties worth the Guinness book. To begin with, the silica-stabilized sp3/sp2 carbon phases demonstrates apparently unique peculiarity previously unknown outside of biological world: a non-monotonic behavior at elevated temperature or under irradiation, including an inverse sp2 → sp3 transition during a short-term exposure, i.e. structural transformation that is opposite to the thermodynamically expected one. (Obviously, this does not mean that DLN "does not respect" the laws of thermodynamics, but, with some modest yet similarity by its complexity to organic world, the fine structure of this non-crystalline system of two interpenetrating networks experiences intrinsic competition between chemical interactions and phase transformation).
The pure DLN carries records with the lowest known secondary electron emission (virtually zero), lowest friction at high humidity or in water (including most severe milieu in the depth of the sea), it has nearly universal adhesion to various inorganic and organic substrates not excluding Teflon; it is stated by the industry that by combination of useful properties and versatility of technical application DLN coatings are the next to none known; besides, they has excellent biocompatibility. Indeed, DLN coatings for medical implants were one of their earliest applications: from 1981, exactly decade before the fundamental structure of DLN with such "given name" had been disclosed in the historically first US patent on Nanocomposites, diamond-like Nanocomposites and, although this term was not used yet in the first patents, but the entire disclosure and model structures - Composites of Atomic Scale. This first patent application submitted to USPTO on May 3, 1991 contains 33 claims, including materials and their structure, method of production and various applications. The USPTO allowed all 33 claims but separated materials, methods and applications in individual patents). In 1980s the coatings saved some human lives, help to many patients and never shown any negative side effect, but unfortunately this work (enthusiastically conducted by closely cooperating researchers and medical doctors without any funds) was interrupted by the outside circumstances.
The pure synergistic graphine - diamond-like QUASAM ( see Fig. 12, a, b) is the only known super-hard solid matter with density on the level of ivory. It is not so good dielectric as DLN, but it has its own practical potential and should be considered as the complementary to DLN synergistic form of carbon.
Fig. 12, a Freestanding graphine mutually/synergistically stabilized with diamond-like network (QUASAM) - samples under loads: 1, 2 – cantilever geometry: 1 – the length l, width w and thickness t of cantilever are correspondingly l=23 mm, w=0.8 mm, t=95 μm; load P=0.02 N. 2 - l=15 mm, w=3 mm, t=95 μm; P=0.05 N. 3,4 – beam geometry, l=25 mm, w=2 mm, t=90 μm: 3 - P=0.2 N. 4 - P=0.05 N. 5, 6 – column geometry, l=12 mm, w=10 mm, t=100 μm, P=1.5 N. 1995, Long Island, NY.
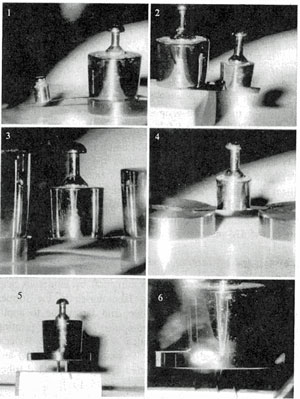
Fig. 12, b The cross-section of hetero-structure: single-crystal silicon (the light left side) and QUASAM (the dark right side) after multiple indentations by diamond pyramid. The splitting and cracks are visible on all imprints in silicon but none in QUASAM: a unique fracture toughness of so hard solid. The indents in Si in vicinity of Si|QUASAM interface produce splitting proliferating into the silicon side but arrested by the interface; most remarkably and surprisingly, imprints precisely targeting the interface did not result with any splitting between Si and QUASAM, although they did result with splitting in silicon. This means that inter-atomic bonds between silicon and QUASAM are stronger than intrinsic bonds in silicon.

This phenomenon is also occurred with various metallic, ceramic and even glass substrates and it has important practical implementations, including so unexpected as certain increase of flexibility of common glass.
As for metal-DLN composites of atomic scale, they carries even greater number of records by the range of conductivity variable in one solid matter, by structural stability of amorphous metals and resistance to millions thermal-electrical shocks in severe milieu, by electronic properties of some {Me-DLN | DLN| semiconductor}-heterostructures and unusual phenomena revealed in low-temperature Me-DLN superconductors.
Materials, as the very base of technological progress, represent its the most conservative field, and a half century is the only beginning of exploration of these novel matters; although this beginning already keeps in memory a lot of dramatic events, hardships and even despair, it did overcome not only physical activation barriers; by the efforts of numerous researchers, engineers, technicians and business entrepreneurs, this technology is broadly employed in both hemispheres for uncounted applications.
Still, the main heroes of this story are working in union carbon, symbolizing the organic nature, and silicon, symbolizing the advanced techno-sphere.
This post is based on the invited chapter for the Millennium Handbook by Academic Press: B.F. Dorfman, Stabilized sp2/sp3 Carbon and Metal-Carbon Composites of Atomic Scale as Interface and Surface-Controlling Dielectric and Conducting Materials. Handbook of Surfaces and Interfaces of Materials (H. S. Nalwa Ed.), v.1, Academic Press, San Diego, 2001, pp. 447-508. The Chapter contains extensive references on the subject up to the year 2000. For the further reading on recent development and applications, the following key words may be used on web: "DLN AND Diamond", "Dylyn AND Diamond", "(alternative trademark for DLN), Diamond-Like Nanocomposite Coating, as well as Bekaert - the international leader of DLN/Dylyn industrial applications.
Acknowledgement
Content of the web page has been developed by Benjamin Dorfman
