Symmetry
Symmetry plays a very important role in nature and there is certainly no exception at the atomic scale. Atoms themselves are highly symmetric (spheres) and most of the molecules and all crystalline materials have some elements of symmetry - centers (inversion), axes (rotation), planes (reflection), translation and a general combination of them all. For example, a spiral; a fascinating feature of the DNA molecule, is a combination of rotation around and translation along the symmetry axis.
Each molecule is assigned to a certain point group: a set of symmetry elements which can be applied to this molecule. Completely asymmetric molecules belong to the group C1, which has only one symmetry element: identity (E). Obviously, all other molecules have identity as well and thus all point groups have element E present.
Here are some examples of well known small molecules which have symmetry:
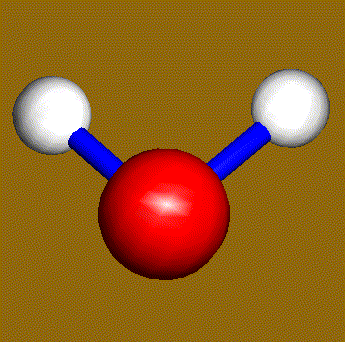
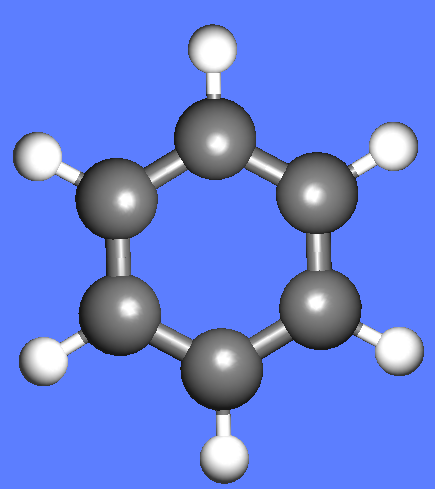
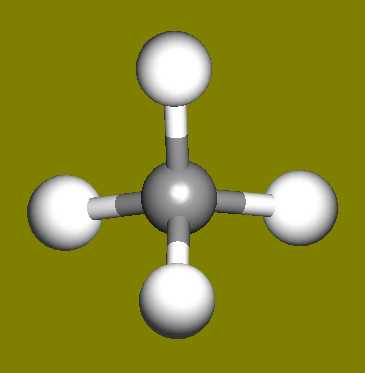
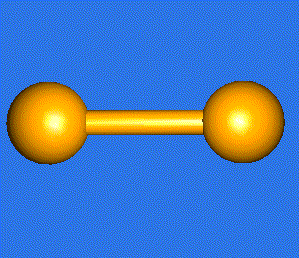
The water molecule (H2O) has four symmetry elements: identity (E), 2nd order axis of symmetry (C2) and two symmetry planes (σv). Higher symmetry molecules, such as benzene (C6H6) and methane (CH4), have more elements of symmetry. Methane has five and benzene has twelve different elements of symmetry, while the total number of symmetry elements: twenty four - is equal for both molecules. Hydrogen, a linear molecule with two equivalent atoms, has an infinite number of symmetry elements such as rotations (C2 and C∞), rotation-reflections (S∞) and planes (Cv).
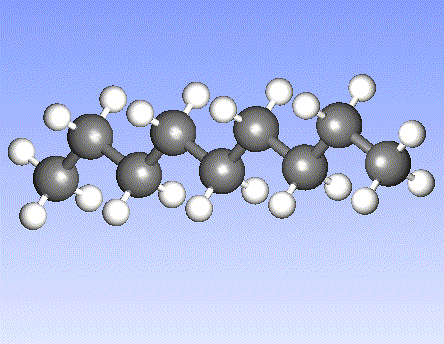
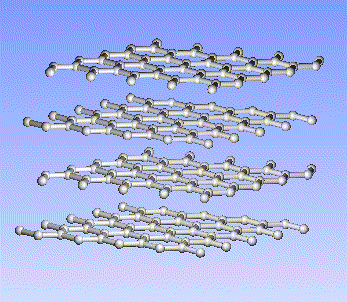
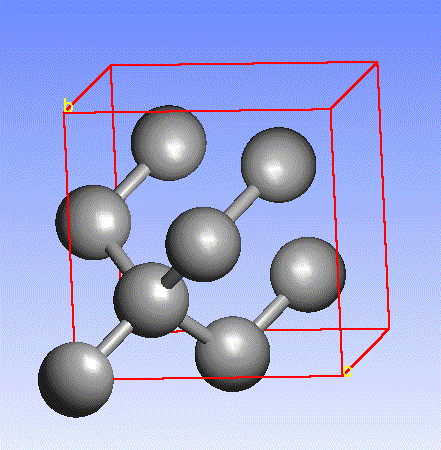
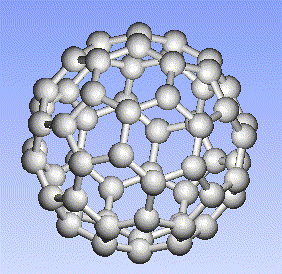
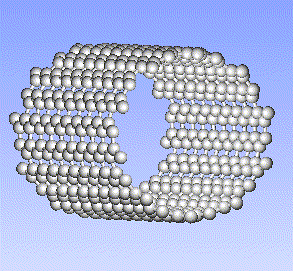
Carbon, the major element in organic molecules, can form linear polymers (such as hydrocarbons (CnHm), two-dimensional sheets (graphite) and three-dimensional crystals (diamond). Fullerenes and nanotubes, which are very popular in nanotechnology research, are also made from carbon as pictured below:
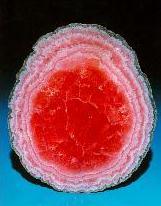
Crystals, macroscopic periodic systems, made from atoms and molecules have fascinating symmetry, which in combination with color is a working horse of the big industry sector - jewelry.Examples of crystals from Steve Smale's collection:
There are fourteen 3D crystal lattices with different symmetries (Bravais lattices): which are characterized by the relations between unit cell parameters and the presence of the additional lattice point in the center of the unit cell or on its faces. See below:
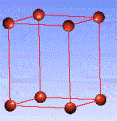
Primitive(P)
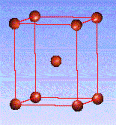
Body-centered(I)
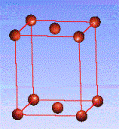
Side-centered(C)
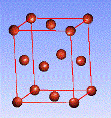
Face-centered(F)
There are seven crystal systems which have one or more different types of unite cell (P, I,C, or F):
Crystal system |
Unit cell coordinates |
Symmetry |
Bravais lattices |
Cubic |
a = b= c; |
Four 3-fold axes |
P, F, I |
Tetragonal |
a = b ≠ c; |
One 4-fold axis |
P, I |
Orthorombic |
a ≠ b ≠ c; |
Three 2-fold axes or mirror planes |
P, F, I, C |
Hexagonal |
a = b ≠ c; |
One 6-fold axis |
P |
Trigonal |
a = b ≠ c; |
One 3-fold axis |
P (R) |
Monoclinic |
a ≠ b ≠ c; |
One 2-fold axis or mirror plane |
P, C |
Triclinic |
a ≠ b ≠ c; |
None |
P |
There are 230 space groups which are made of 32 crystallographic point groups (crystal classes) compatible with translation symmetry.
Acknowledgement

This web page is prepared by Anatoli Korkin. Numerous references have been provided by Dr. Inbal Tuvi-Arad. Atomic scale images are created with SageMD2 program and DNA image is created by Anastassia Alexandrova.
More information on molecular and crystal symmetry
- Molecular Symmetry Online by Inbal Tuvi-Arad, The Open University of Israel
- Continous Symmetry Measures by Inbal Tuvi-Arad, The Open University of Israel
- Molecular Symmetry: An Alternative Guide
- Symmetry Resources at Otterbein College
- 3DMolSym – Symmetry Visualization Software
- Chirality Game at Nobelprize.org
- Introduction to Crystallography
- Mineralogy Data Base
- Crystal Lattice: Structures
- Snow Crystals and Snowflakes
- Bilbao Crystallographic server
- Cambridge Crystallographic Data Center